Body-on-a-Chip and Tissue Culture in Microfluidic Devices
|
Body-on-a-Chip is an emerging research area that cultures human organ cells in microfluidic devices, growing 3D tissues that accurately mimic the structure and function of human organs. Different tissue types, such as lung, heart, liver, brain, kidney, and intestines, are cultured in compartmentalized microenvironments, representing organs. Then the various tissue types are connected through fluidic conduits representing a synthetic circulatory system. The result is an integrated body-on-a-chip with the ability to model the complex functions of the human body.
By replicating the smallest possible functional units of multiple human organs on microdevices, the goal is to develop the best predictive model of human response to therapies, drugs, cosmetics and other chemical compounds. This is an important new area of development because the current cell and animal models used to predict human response to chemical compounds are inaccurate. The limitations of these cell and animal models become obvious when considering the high failure rate of new drug therapies in the FDA approval process. Upwards of 85% of drug candidates, that seem promising from pre-clinical studies, fail in human clinical trials due to limitations in safety and effectiveness. Equally alarming are the possible life-saving therapies that were abandoned and never entered human clinical trials because they proved toxic by the inaccurate models. Therefore improved, predictive models of human response to chemical compounds are needed. |
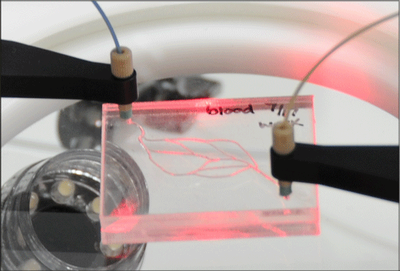
 The PeriWave flow control pump allows for culture medium to be continuously circulated for the duration of an experiment.
The PeriWave flow control pump allows for culture medium to be continuously circulated for the duration of an experiment.
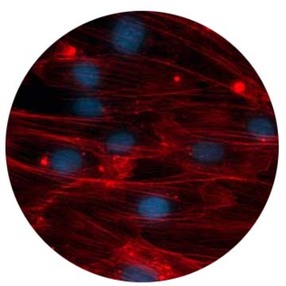
By culturing actual human tissues, Body-on-a-Chip offers a more accurate, more efficient and kinder alternative to animal testing. Due to fundamental biological differences, animals are simply not good analogues for humans. Furthermore Body-on-a-Chip offers an improved alternative to conventional 2D cell culture. In conventional assays, cells are cultured in a two-dimensional monolayer, immersed in excess medium. In contrast, cells in native 3D tissue are surrounded by extracelluar matrix and other supporting cells. This drastically different cell microenvironment between 2D cell-based and 3D tissue assays results in conventional 2D cell-based assays inadequately modeling the nutrient transport, shear stress, and both chemical and mechanical signaling processes of cells.
Microfabrication allows for devices with precisely tailored geometries and flow patterns, resulting in controllable, reproducible and more in vivo-like microenvironments. Cells cultivated in microdevices adhere to the walls, allowing the perfusion of medium inside the tissue. This both improves metabolic waste removal and continually renews nutrient supply, creating a more physiological-like situation. However, in order to achieve these desirable microenvironments, researchers must heed special attention to how the growth factors and reagents are transported inside the microdevice. In particular, the sheer force applied to the cells by the “blood” or culture medium circulating through the Body-on-a-Chip device has been shown by numerous groups to affect cell elongation, alignment, and more than likely, function.
Two high performance flow control pumps that have been successfully employed in 3D tissue culture experiments are the PneuWave and PeriWave. The PneuWave is a pneumatic, pulseless pump with both flow rate and pressure closed-loop feedback control. With an internally integrated compressor, user-friendly software, and fast response, this accurate fluid delivery pump is ideal for Body-on-a-Chip experiments. Likewise, the PeriWave is also well suited for this application. The PeriWave has peristaltic-based fluid delivery with an integrated flow sensor for closed-loop feedback control. This pump can both push and pull fluid accurately with fast response times and no pulsation. Furthermore the PeriWave is uniquely able to aspirate and dispense from the same fluid reservoir, allowing culture medium to be recirculated throughout a microdevice for weeks or longer.
Body-on-a-chip is an exciting area, and though in its infancy, has already demonstrated great potential for offering faster, cheaper and more accurate prediction of a drug’s in vivo pharmacokinetics and pharmacodynamics. If the approach demonstrates it can successfully predict drug efficacy and safety in humans, the field could transform how therapies are developed, how clinical trials are conducted, and the regulatory decision-making process.
Microfabrication allows for devices with precisely tailored geometries and flow patterns, resulting in controllable, reproducible and more in vivo-like microenvironments. Cells cultivated in microdevices adhere to the walls, allowing the perfusion of medium inside the tissue. This both improves metabolic waste removal and continually renews nutrient supply, creating a more physiological-like situation. However, in order to achieve these desirable microenvironments, researchers must heed special attention to how the growth factors and reagents are transported inside the microdevice. In particular, the sheer force applied to the cells by the “blood” or culture medium circulating through the Body-on-a-Chip device has been shown by numerous groups to affect cell elongation, alignment, and more than likely, function.
Two high performance flow control pumps that have been successfully employed in 3D tissue culture experiments are the PneuWave and PeriWave. The PneuWave is a pneumatic, pulseless pump with both flow rate and pressure closed-loop feedback control. With an internally integrated compressor, user-friendly software, and fast response, this accurate fluid delivery pump is ideal for Body-on-a-Chip experiments. Likewise, the PeriWave is also well suited for this application. The PeriWave has peristaltic-based fluid delivery with an integrated flow sensor for closed-loop feedback control. This pump can both push and pull fluid accurately with fast response times and no pulsation. Furthermore the PeriWave is uniquely able to aspirate and dispense from the same fluid reservoir, allowing culture medium to be recirculated throughout a microdevice for weeks or longer.
Body-on-a-chip is an exciting area, and though in its infancy, has already demonstrated great potential for offering faster, cheaper and more accurate prediction of a drug’s in vivo pharmacokinetics and pharmacodynamics. If the approach demonstrates it can successfully predict drug efficacy and safety in humans, the field could transform how therapies are developed, how clinical trials are conducted, and the regulatory decision-making process.